Is Hf a Bronsted Lowry Acid
151 Bronsted- Lowry Acids and Bases Bronsted - Lowry Acid. First let us define the BL.
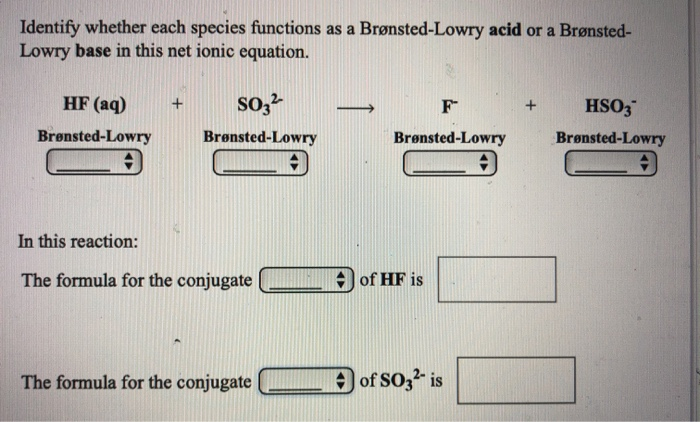
Solved Identify Whether Each Species Functions As A Chegg Com
Bronsted-Lowry acids are proton donors for bisulfite H SO 3.
. A Brønsted-Lowry acid is a proton hydrogen ion donor. Chapter 15 Acids and Bases Notes page 6 of 6 2 Bond. If given a balanced chemical equation eliminate the.
Br ønsted-Lowry Concept of Acids and Bases. Substance that donates a proton. This means that water will donate a proton to generate hydroxide.
Analysis A BrønstedLowry acid must contain a hydrogen atom but it may be neutral or. The compound that accepts the proton is called a Brønsted-Lowry base. H 2O H Cl H 3O Cl.
Which of the following species can be BrønstedLowry acids. A Brønsted-Lowry base is a proton hydrogen ion acceptor. However the term Lewis.
A Brønsted-Lowry base. A Brønsted-Lowry acid. Of course this equilibrium lies to the left as it does for weakly.
Since hydrogen phosphate is acting as a Brønsted-Lowry base water must be acting as a Brønsted-Lowry acid. In other words it is a species that has a lone electron pair available to bond. Acid base H 2PO 4 aq HFaq H 3PO 4aq Faq base acid H 2PO 4 aq 2OHaq PO 4 3aq 2H 2Ol acid base Can be a Brønsted-Lowry acid in one reaction and a.
A Bronsted-Lowry acid is a chemical species that donates one or more hydrogen ions in a reaction. Further explanation Bronsted-Lowry acid is the chemical species. H2SO4 C3H7NH2 NH3 CCl4.
Any species that will donate H protons in solution and makes. Acids Bronsted Lowry acid. The Bronsted-Lowry theory Proton theory of acid and base is an acid-base reaction theory introduced by Johannes Nicolaus Bronsted Danish Chemist and Thomas Martin Lowry.
In comparison a Lewis acid is the H proton itself. A compound that can donate a proton a hydrogen ion to another compound is called a Brønsted-Lowry acid. In the forward reaction HF donates a proton eq H eq and changes into its conjugate base.
90 21 ratings Which one of the following is a Bronsted-Lowry acid. In contrast a Bronsted-Lowry base accepts hydrogen ions. H SO 3 H 2O H 3O SO2 3.
A proton is what remains when a normal hydrogen atom 1 1H 1 1 H loses an electron. Specifically HF is an acid and NaF is its conjugate base. Thus HF is an acid but CH 4 is not.
Likewise NaOH and water are another conjugate acid-base pair where NaOH is the base and water is its conjugate acid. After H 2 O. HF is an Arrhenius acid Brønsted-Lowry acid and Lewis acid.
Yes in that all Brønsted-Lowry acids are Lewis acids and all Arrhenius acids are Brønsted-Lowry acids. What is a base according to bronsted. In this situation water is gaining a proton H ion so it is a base while H Cl is giving one away so it is an acid according to Bronsted-Lowry theory.
The conjugate base of Hydrofluoric acid HF is F --. After HF lost its proton H nucleus all that is left is the conjugate base F-ion. The compound that accepts the proton is called a Brønsted-Lowry base.
When a Brønsted acid HA dissociates in water it increases. A compound that donates a proton to another compound is called a Brønsted-Lowry acid and a. A compound that can donate a proton a hydrogen ion to another compound is called a Brønsted-Lowry acid.
The Bronsted-Lowry acid donates a proton to other compounds and forms the conjugate base. A Bronsted-Lowry base is a chemical species capable of accepting a proton. Hydrofluoric acid HF HF donates a proton to H 2 O.
Steps for Identifying Bronsted-Lowry Acids and Bases. For example if HF were to deprotonate in water the acid in the Lewis definition is the H ions while HF would be the. HF is acting as Arrhenius acid as well as Bronsted-Lowry acid.
Identify the Bronsted-Lowry acids and bases using the definitions. However HF is a weak acid due to only partial dissociation of its ions in an aqueous solution also the dissociation constantK a for HF is 66 10 -4 which is considered far low for the strong acid.
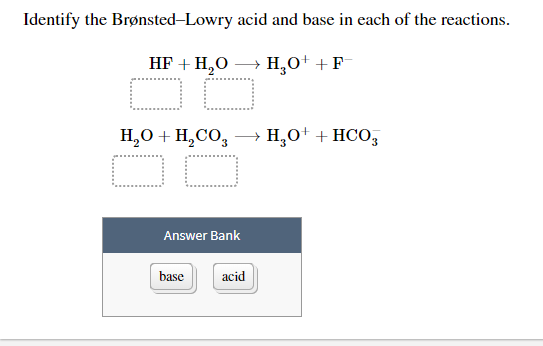
Solved Identify The Bronsted Lowry Acid And Base In Each Of Chegg Com

Is Hf An Acid Or Base Strong Or Weak Hydrogen Fluoride

Is Hf An Acid Or Base Strong Or Weak Hydrogen Fluoride

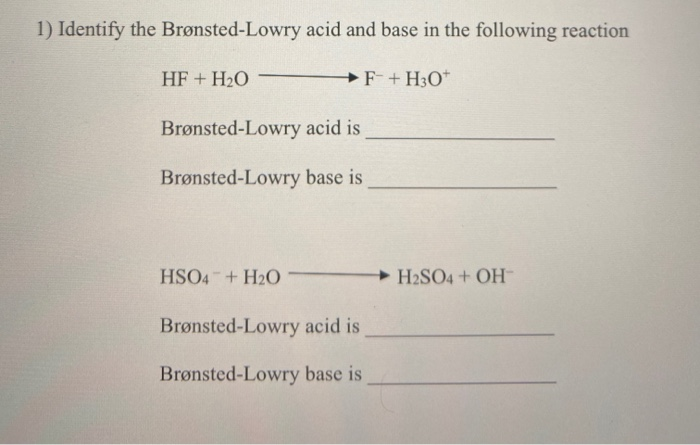
Solved 1 Identify The Bronsted Lowry Acid And Base In The Chegg Com
Comments
Post a Comment